White Papers
Solubility²

Together with the Solubility Company Oy (Helsinki, Finland) we explored the potential of their revolutionary small-scale SPA™ technique for solubility measurements as input for the PC-SAFT parametrization of pharmaceuticals.
Download the white paper to see how good the combination is!
Formulation²
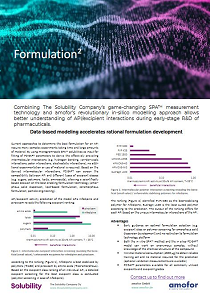
A negligibly small amout of material is sufficient for characterizing intermolecular interaction profiles of an API. The Solubility Company Oy (Helsinki, Finland) determined with their revolutionary small-scale SPA™ technique solubilities in organic solvents, serving as input for a PC-SAFT based predictive formulation screening at amofor.
Find out already at early stage what type of enabling formulation (e.g. lipid, ASD, co-amorphous) and even which excipient type is the best for your API, whithout the need of further experiments!
ASD shelf life

The crystallization onset time in a metastable ASD is crucial for determining the shelf life of an ASD.
Many variable factors affect the true shelf life of an ASD, making a prediction for any storage humidity, temperature or API load almost rpedictable. Have a look at this white paper and learn more about our fast-track revolutionary approach to circumvent this issue!
Big Data meets Physics

Explore the power of big data to revolutionize your drug formulation process. With amofor’s physics-based simulations, you can predict solubility behavior and molecular interactions with precision, reducing trial-and-error and accelerating your R&D efforts.
Explore how big data combined with powerful physical modeling opens a new door in formulation development.
